Umbilical Cord Mesenchymal Stem Cells (UC-MSC) Trials Set for Life-Saving Treatment to Prevent Lung Damage, Organ Failure
FOR IMMEDIATE RELEASE:
Top scientists, researchers, and innovators from The Cure Alliance, a U.S.-based non-profit 501(c)(3) organization, together with its sister organization in Italy, Fondazione Cure Alliance ONLUS, are launching an international effort for the clinical testing of a cellular therapy for a promising new treatment for life-threatening cases of Coronavirus Disease 2019 (COVID-19). The therapy uses cells from discarded umbilical cords, with just one cord saving as many as 10,000 lives.
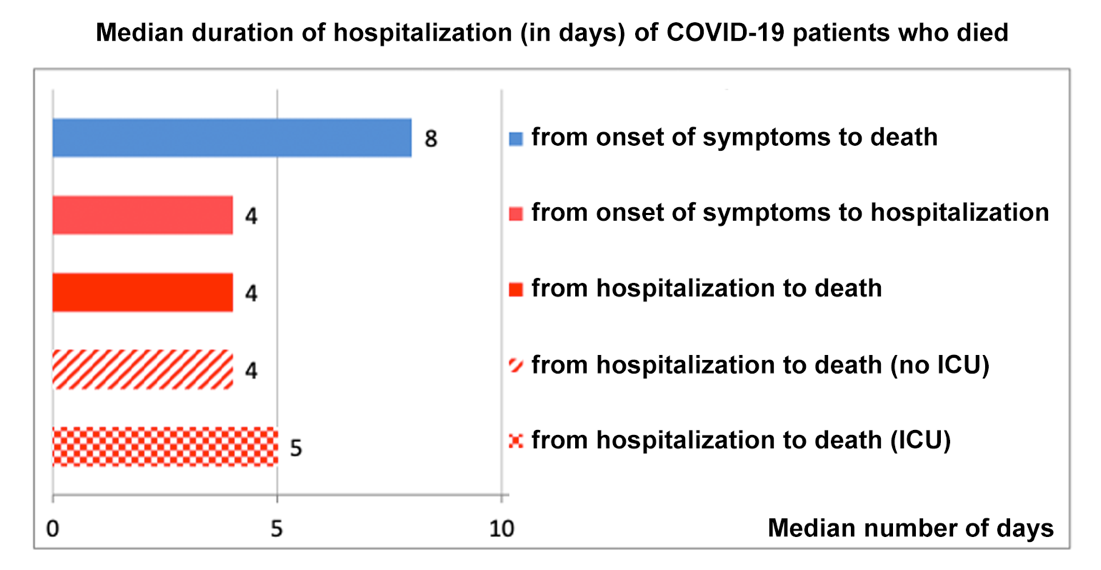
Why people die from COVID-19 and pneumonia: a highly contagious virus causes, in many people, severe lung inflammation, making breathing difficult. Oxygen levels in the bloodstream drop and vital organs begin to fail. There is no time to waste: the average time between first symptom and death from COVID-19 is just 8 days.
(translated from https://www.epicentro.iss.it/coronavirus/aggiornamenti; March, 17 2020)
The science suggests the severe lung inflammation can be blocked with a simple IV infusion of Umbilical Cord Mesenchymal Stem Cells (UC-MSCs), preventing the life-threatening progression of COVID-19.
Pre-clinical and early clinical testing in pilot clinical trials indicate that this cell type can have a significant life-saving effect on patients affected by the most severe cases of COVID-19, associated with a powerful inflammatory reaction and associated cytokine storm leading to Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS).
WHAT WE NEED: We are seeking support for rapid expansion of clinical grade (cGMP) UC-MSC Master Cell Bank product manufacturing and for clinical trials in patients with severe cases of COVID-19. These cell products have been already approved by the FDA for testing in Diabetes and Alzheimer’s and we are now expanding their indication for clinical testing in COVID-19.
Resources are urgently needed to expand the manufacturing capabilities and make sure we will have sufficient cell product to support its clinical use in COVID-19. Once the pandemic is resolved, excess cell product will remain in the Master Cell Bank to be used for research and clinical trials in Diabetes, Alzheimer’s and Pulmonary Fibrosis.
This proposed cellular therapy represents a collaborative, international, academic, non-profit initiative, sponsored by The Cure Alliance and Fondazione Cure Alliance ONLUS. This newly-provided therapy will be provided at no charge.
THE ALL-STAR TEAM: We encourage you to scroll through the biographies of the elite scientists and academics on the international multidisciplinary team directly involved with infectious disease, regenerative medicine, pulmonary medicine and critical care. Our team also has extensive experience with cell-based products development, characterization, and utilization in clinical trials.
Camillo Ricordi, MD, Stacy Joy Goodman Professor of Surgery, Distinguished Professor of Medicine, Professor of Biomedical Engineering, and Microbiology and Immunology at the University of Miami (UM), Florida, where he serves as Director of the Diabetes Research Institute (DRI; www.diabetesresearch.org) and the Cell Transplant Center. Dr. Ricordi is recognized as a world leader in immunotherapy, cell replacement therapy and islet cell transplantation. He has been serving as Head of the NIH funded cGMP (current Good Manufacturing Practices) Advanced Cell and Biologic Product Manufacturing Facility (1993-present), for research and clinical applications at UM, in the US and worldwide. Dr. Ricordi is a fellow of the National Academy of Inventors, USA. He is a member of the Italian Supreme Council of Health. He has a vast experience in cellular therapies and has led and participated a number of clinical trials in patients with Type 1 Diabetes, conducted over the last 30 years. Dr. Ricordi will oversee, steer, and manage the overall direction of this study, will be accountable for key tasks and study deliverables. Dr. Ricordi will lead a team of investigators at the University of Miami – Miller School of Medicine.
Marilyn Kay Glassberg, MD, is Professor of Medicine at the University of Arizona College of Medicine is the Chief, Division of Pulmonary Medicine, Critical Care, and Sleep; Vice-Chair of Diversity and Inclusion and Senior Director of Research Strategy and Growth. She is a leading national and international expert in rare lung diseases and idiopathic pulmonary fibrosis. Dr. Glassberg is a physician scientist focused on the role of age and sex in irreversible fibrotic and chronic lung diseases. Her research also involves studies focused on the mechanism of stem/stromal cell-based therapy in these incurable lung diseases.
Roger Alvarez, MD, is Assistant Professor of Medicine at the University of Miami Miller School of Medicine, where his focus is translational research involving advanced lung diseases, pulmonary arterial hypertension and interstitial lung disease (ILD). His clinical work is dedicated to leading multidisciplinary teams to care for people cardiopulmonary diseases including high-risk pulmonary embolism and interstitial lung disease, and caring for critically ill patients in the intensive care unit, with special interests in acute lung injury and shock. Dr. Alvarez completed his medical training at Emory University, and went on to train in Pulmonary & Critical Care Medicine at the University of Pittsburgh. There he gained experience in the management of severe ARDS under the mentorship of program directors Drs. John Kreit and Bryan McVerry, and completed training in translational pulmonary vascular research under the mentorship of Mark Gladwin, MD and Adam Straub, PhD. At the bench, Dr. Alvarez made the novel observation that pulmonary endothelial cells express increased levels alpha hemoglobin in patients as well as cellular and animal models of pulmonary hypertension. He found that endothelial hemoglobin expression mediates part of the endothelial dysfunction and NO depletion seen in hypoxic pulmonary hypertension. After joining the faculty at the UMMSM, Dr. Alvarez continued his research in NO under the mentorship of Dr. Marilyn Glassberg. He brought his NO research to the bedside by completing a study delivering inhaled NO to patients with ILD and pulmonary hypertension, demonstrating significant improvements in hemodynamics during an acute-dose escalation study, where he personally performed many of the hemodynamic studies in the ICU, directing a team of critical care nurses and study staff. He is also PI of several industry-sponsored trials of novel drugs for the therapy of pulmonary arterial hypertension.
Antonio C. Marttos, MD, is Associate Professor of Surgery, Director of Global e-Health/Trauma Telemedicine, Co-Director of the William Lehman Injury Research Center, Ryder Trauma Center- Jackson Memorial Hospital -University of Miami. Dr. Marttos is a pioneer in Trauma Telemedicine has been involved in numerous studies and clinical activities for the Department of Defense, the U.S. Department of State, and the Florida Department of Health. He created a statewide Trauma Telemedicine Network and received the health department’s Outstanding Leadership Award and also the First Annual State Surgeon General Health Innovation, Prevention, and Management Award for these efforts. He is also deeply involved in developing telemedicine solutions to provide expert support in multiple trauma environments, including the Resuscitation and Intensive Care units, the operating room, pre-hospital and mass casualty. He led the Telemedicine Education and Advice for Military Medicine (TEAMM) project, which linked the Ryder Trauma Center with the U.S. Air Force, and has explored the use of telemedicine in mass casualty exercises with the U.S. Army Forward Surgical Teams, as well as in exercises conducted at multiple hospitals across Florida.
Most recently, Dr. Marttos has created an unparalleled Global Telemedicine program funded by the Department of State, to provide trauma telemedicine support services in Iraq. The program has received national attention and is now considered the gold standard for such programs. Dr. Marttos is the President Elect of the Panamerican Trauma Society and currently launched 2 Medical simulations courses that have been implemented around the world ” Critical Decisions in Trauma and Critical Decisions in Medical Emergencies”. He is a full Time Trauma Surgeon d al Critical Care Physician at University of Miami/Jackson Memorial Hospital system.
Jose G. Castro, MD, is a Professor of Clinical Medicine in the Division of Infectious Diseases at the University of Miami Miller School of Medicine. He practices clinical medicine and has been involved in clinical research in the field of infectious diseases for almost 20 years. He is also a member of the University of Miami IRB and is familiar with rules and regulations to conduct research under state and federal guidelines. He is a key member of the University of Miami response team to the current COVID-19 epidemic and also he is part of the teams that will directly interact with patients with this infection.
Amit Patel, MD, is an international leader in innovation and translational of novel biological and minimally invasive therapies for Lung and Heart Disease. He is the past Chief of Cardiac Surgery at the University of Miami Health System and Tenured Professor of Surgery in the Division of Cardiothoracic Surgery at the University of Utah School of Medicine and Director of Clinical Regenerative Medicine and Tissue Engineering at the University of Utah. Dr. Patel has created new stem cell, exosome, genetic, and matrix therapies for lung and heart diseases. He has served as national and international principal investigator in several first-in-human trials approved by the U.S. FDA and has over 200 patents, publications, and national and international presentations. His clinical focus includes advanced heart surgery for coronary disease, valve repair and replacement, heart failure, thoracic aortic surgery and endografts along with minimally invasive thoracic oncology. . His international outreach program for patients with no medical options includes South America, Europe and Asia. He has numerous clinical sites around the world to provide advance cardiac and pulmonary therapies. Dr. Patel received M.D. from Case Western Reserve University, and has a MS in Immunology/Virology. He did his internship and residency in surgery at Baylor University Medical Center, and completed a fellowship in cardiothoracic surgery at the University of Pittsburgh.
Michael J. Paidas, MD, is Professor and Chair, Department of Obstetrics, Gynecology & Reproductive Sciences at the University of Miami Miller School of Medicine, and Chief of Service at University Health Tower and Jackson Health System. Dr. Paidas re-established the Maternal Fetal Medicine Fellowship Program of the University of Miami-Jackson Health System. Dr. Paidas arrived at the UMMSOM in November 2018, following a 16-year career at the Yale Medical School, where he held several positions including Professor and Vice Chair, Obstetrics and Director of the Maternal Fetal Medicine Fellowship. Dr. Paidas was also Director of the Yale Women and Children’s Center for Blood Disorders & Preeclampsia Advancement, and Co-Director of the National Hemophilia Foundation-Baxter Clinical Fellowship at Yale. Dr. Paidas served as Interim Director of the Maternal Fetal Medicine Section, and Interim MFM Section Chief of Yale New Haven Hospital.
JoNell E. Potter, PhD, is Professor of Clinical Obstetrics and Gynecology at the University of Miami, Miller School of Medicine, with joint appointments in the Department of Pediatrics and School of Nursing. Dr. Potter also serves as the Vice Chair for Reproductive Sciences, and Chief of the Women’s HIV Service at Jackson Health System. She has served as an Investigator on multiple, federally funded research projects and has authored and co-authored manuscripts, book chapters, presenting her work at national and international meetings. In her role as Vice Chair for Reproductive Sciences, she will assist the PI and project team with specimen collection and other aspects of this trial to ensure the goals of the project are met.
Jianming Tan, MD, PhD, is Professor of Surgery at Fuzhou General Hospital, Xiamen University, China. Dr. Tan serves as Vice President of Fuzhou General Hospital, Xiamen University and Director of Cell and Organ Transplant Institution of the PLA. Dr. Tan also serves as Director of the Fujian Key Laboratory of Transplant Biology and Director of Department of Urology of Fuzhou General Hospital. Dr. Tan is a founding member of The Cure Alliance and is a a world-renowned surgeon scientist with over 30 years of experience focusing on translational and clinical research projects in the field of transplant immunobiology, autoimmunity, immunotherapy, regenerative medicine, stem cells, and tissue engineering. In recent years, Dr. Tan has led a number of clinical trials on the use of UC-MSC cellular therapies in organ transplantation, autoimmunity, diabetes mellitus, and is now leading pilot efforts of UC-MSC infusion in severe cases of COVID-19.
Giacomo Lanzoni, PhD: has 15 years of experience on placenta- and umbilical cord-derived MSCs. He has extensive experience in the development and characterization of MSC and stem cell products for cell therapy. His main interest is on the clinical translation of the immunomodulatory and regenerative properties of MSCs. He will be responsible for the establishment of the UC-MSC primary culture for the generation of the UC-MSC MCB. Moreover, he will be responsible for the characterization of the UC-MSC immunomodulatory potency, both in vitro and in treatment recipients.
Diego Correa, MD, MSc, PhD: has 15+ years of experience conducting pre-clinical and clinical research in the Regenerative Medicine field. He has experience with in vitro and in vivo animal models to test efficacy of cell-based therapy products obtained from multiple different sources (bone marrow, adipose tissue, infrapatellar fat pad, endometrium, umbilical cord) and manufactured under different conditions including regulatory-compliant protocols. He has developed potency assays testing MSC’s secretory and immunomodulatory activities, based on the description of phenotypic and functional readouts. Additionally, he has directed clinical teams abroad focused on establishing safety and efficacy of cell-based products (point of care and culture-expanded ones) in a number of clinical conditions including peripheral vascular disease (PVD), chronic kidney disease (CKD) and osteoarthritis (OA). Along with Drs Lanzoni and Linetsky, Dr Correa will contribute to the cell characterization of UC-MSC before their clinical use in patients enrolled in this study.
Elina Linetsky, PhD: will generate the UC-MSC MCB. Dr. Linetsky has been working with the MSC products for the last 15 years, and is considered to be an expert in the field of cellular therapies. She has extensive experience in cellular therapy manufacture and regulatory product development. Over the years, the cGMP Advanced Cell and Biologic Products Manufacturing Facility under Dr. Linetsky`s leadership has supported a number of DRI-, UM- and extramurally initiated clinical trials via cellular product manufacture, characterization, and regulatory product development. The Facility personnel are experienced in the manufacture, characterization, storage and distribution of cellular therapies, particularly MSC products from various sources.
Khemraj Hirani, PhD, MBA: has worked for more than ten years to provide guidance and direction for clinical operations and research while performing oversight for regulatory compliance, quality assurance, and controls. He is recognized as a subject matter expert on FDA and CFR regulations related to clinical trials in endocrinology, HIV/AIDS, cancer, CNS, Biologics, cardiovascular and infectious diseases, vaccines, and safety updates. To date, he has conducted thousands of successful clinical trials and has led teams of clinicians and support staff in those efforts. His areas of expertise include: Drug Development, Phase I to IV Trials, ICH/GCP Guidelines and FDA Regulations, Regulatory Dossier, Multiple Therapeutic Areas, Design of Research Protocol, Research Compliance, Data Safety Monitoring, Human Subject Protection, Pharmacovigilance, and Drug Safety.
David Baidal, M.D. Dr. Baidal’s clinical training and research background provides him with unique qualifications to successfully carry out the goals of this clinical trial. He obtained his MD at the Universidad Católica Santiago de Guayaquil in Ecuador in 2000m and joined the Clinical Islet Transplant Program (CITP), DRI, UM as a post-doctoral fellow in 2001. His responsibilities included recruitment of individuals with uncontrolled T1D for participation in clinical trials of islet transplantation, monitoring and performing clinical evaluations of participants post islet transplantation, conducting metabolic testing and evaluating metabolic markers of β-cell function and reserve, as well as several regulatory activities namely, reporting of adverse events to regulatory entities, updating study protocols and consent forms, and collaboration in the development of yearly clinical reports. He successfully collaborated with other researchers and chaired the Transplant Coordinators/Data Managers committee for the Collaborative Islet Transplant Registry (CITR) during its inaugural year and was an active member of several CITR committees. He completed his internal medicine training at Jackson Memorial Hospital, UM in 2012 and his clinical and research fellowship in Endocrinology, Diabetes and Metabolism at the Beth Israel Deaconess Medical Center and Joslin Diabetes Center, Harvard School of Medicine in 2015. He re-joined the CCTP, DRI, UM in 2017 and became the Center Director for the TrialNet Clinical Center at the DRI, UM. He is currently involved with the management of islet transplant recipients, development of clinical trials aimed at optimizing islet transplantation and testing novel cellular and immunomodulatory therapies. Current clinical trial builds logically on Dr. Baidal’s prior clinical and research experience and he is an integral member of the UM-DRI clinical research team.
Rodolfo Alejandro, MD, study Co-PI, has >30 years clinical trial experience in T1D immunotherapies and islet transplantation. Working closely, the PI and Co-PI will assure that all personnel involved in the study are appropriately trained, the project is initiated and conducted as outlined in the project-specific institutional SOPs and applicable regulations. PI mentors will serve as scientific advisors. Regular meetings will be held on regular basis as the project develops, to deal with problems as they arise. Study PI, Co-PI and mentors will assure smooth conduct of the project.
Xiumin Xu, MSc: is the Director of the China-USA Collaborative Human Transplant Program at the Diabetes Research Institute (DRI) at University of Miami, Leonard M. Miller School of Medicine. She serves as Director of Quality Assurance and Regulatory Affairs Quality Control/Assurance of cGMP Cell Processing and Transplant Center at Fuzhou General Hospital, Xiamen University, China. She also served as Supervisor of the state-licensed Clinical Flow Cytometry Laboratory (2004-2013). She has more than 25 years of laboratory experience, primarily in human cells and tissues processing, including but not limited to hematopoietic cells from vertebral body marrow, mobilized peripheral blood, cord blood, iliac crest aspirates bone marrow; mesenchymal stem cells from fat, bone marrow, umbilical cord, and placenta; islets from cadaveric pancreas and autologous donors. Her area of interest is translational and potential clinical applications in the field of transplant immunology and regenerative medicine.
Bradley J. Goldstein, MD, PhD is Associate Professor of Head and Neck Surgery & Communication Sciences and Vice-Chair of Research, Duke University School of Medicine, Durham, NC, USA. He is a surgeon-scientist focused on development of new therapies for disorders of olfaction. His research involves investigating mechanisms regulating regeneration and tissue homeostasis in adult olfactory mucosa in humans and mouse model
Proposal Summary
Why people die from COVID-19 and pneumonia: a highly contagious virus causes a severe lung inflammation, in some people, making breathing difficult. Oxygen levels in the bloodstream drop and vital organs begin to fail. There is no time to waste: the median time between first symptom and death from COVID-19 is just 8 days.
The CIRCLE OF LIFE Project: We propose to block the severe lung inflammation with a simple intravenous infusion of Umbilical Cord-derived Mesenchymal Stem Cells, to prevent the life-threatening progression of the disease COVID-19.
THE CURE ALLIANCE “CIRCLE OF LIFE” LEADERS CAN OUT-PACE OTHER TRIALS AND WILL SHARE KNOWLEDGE, ACCELERATING TREATMENT TO PATIENTS.
DONATE NOW AND HELP US SAVE LIVES https://www.thecurealliance.org
The cell product used in this clinical trial will be Umbilical Cord Mesenchymal (Stromal) Stem Cells (UC-MSC). Preclinical and early clinical testing in pilot clinical trials have been promising and indicate that this cell type could have a significant life-saving effect on patients affected by the most severe cases of COVID-19, who are undergoing cytokine storm leading to Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS).
We are seeking support to rapidly expand perform pilot testing (compassionate use) and make available clinical grade (cGMP grade) UC-MSC Master Cell Bank (MCB) products, for treatment of COVID-19 patients with such severe complications. These cell products have been already approved by the FDA for testing in Diabetes and Alzheimer’s and we are now expanding their indication for pilot testing in COVID-19.
Resources are urgently needed to expand the manufacturing capabilities and make sure we will have sufficient cell product to support its clinical use in COVID-19. Should the pandemic be resolved, excess cell product remaining in the Master Cell Bank will be transferred for use in research and clinical trials for Diabetes, Alzheimer’s and Pulmonary Fibrosis.
Contributions can be made through TheCureAlliance.org DONATE button,
or directly to:
The Cure Alliance 501(c)(3) – Tax Exempt for Tax Deductible donations)
For Domestic (USA) Wires:
Wire Routing Transit Number (RTN/ABA) 121000248
For International Wires:
SWIFT/BIC code WFBIUS6S
Bank name: Wells Fargo Bank, N.A.
Bank address: 420 Montgomery city & state San Francisco, CA 94104 (regardless of where your account is located) BNF/Field 4200
FUNDRAISING GOAL: $5,000,000 (the entire amount raised will support the proposed clinical trials, with no indirect cost or administrative fee charged by The Cure Alliance or Fondazione Cure Alliance ONLUS). This funding will allow for cell product processing, quality controls, patient treatment and follow-up mechanistic studies for up to 100 patients. Once safety and efficacy of this therapeutic strategy are determined, further scale up of the operation will require a substantially lower cost per patient.
Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) recognize the sites of injury, limit the cytokine storm associated with severe immune reactions, and stimulate tissue regeneration. Importantly, UC-MSCs have been reported to inhibit inflammation and fibrosis in the lungs. For these reasons, UC-MSCs and have been recently suggested for the treatment of patients with severe COVID-19. UC-MSCs can be easily derived in large numbers from the Umbilical Cord, can be rapidly expanded into clinically-relevant numbers, and have demonstrated safety and efficacy in clinical trials.
UC-MSCs are routinely processed at the Diabetes Research Institute Cell Transplant Center (cGMP Advanced Cell and Biologic Product Manufacturing Facility). Besides collaborative international trials to prevent the progression of chronic organ failure in diabetes, these cells have been recently approved by FDA for administration in patients with Alzheimer’s and Type 1 Diabetes.
We believe that UC-MSC may represent a viable therapeutic option for severe cases of COVID-19, associated with cytokine storm leading to ALI/ARDS. We propose to rapidly expand our existing cGMP grade Master Cell Bank to offer UC-MSC products in the fastest, most efficient and safest way possible, to treat the rapidly growing population of COVID-19 patients with such severe complications.
CELLS FROM JUST ONE UMBILICAL CORD COULD SAVE 10,000 LIVES.


We are a Stem Cell Institute based in Miami for over 15 years and would love to help with any pending trials etc. Please contact me directly at: orodriguez@miamistemcell.co to see how we can help you! We have plenty of Bilogical Umbilical Cord products and also conduct Bone Marrow Stem Cell Transplantation in our own clinic with a highly acclaimed team of doctors.